Any information processing scheme that relies on the precise timing of action potentials obviously requires a means to transmit spikes without destroying their temporal structure. A critical question is thus whether a packet of initially synchronous action potentials can be transmitted from one brain area to the next without loosing the information. In this section we show that packets of (almost) synchronous spikes can propagate in a feed-forward structure from one layer to the next in such a way that their degree of synchrony is preserved - despite the presence of noise in the spike generating mechanism. Moreover, the temporal dispersion within such a packet can even be reduced during the transmission. This results in a stable wave form of the spike packet that can propagate - very much like a soliton - through the network (Kistler and Gerstner, 2001; Diesmann et al., 1999; Gewaltig, 2000).
The phenomenon of a stable propagation of synchronous spikes has been proposed by M. Abeles as an explanation for precisely timed spike events in multi-electrode recordings that seem to occur with a frequency that is incompatible with purely random (Poisson) spike trains; but see Oram et al. (1999). He suggested that neurons that participate in the transmission of these spikes form a so-called `syn-fire chain' (Abeles, 1991). More generally, the propagation of (partially) synchronous spikes is expected to play a role whenever information about a new stimulus has to be reliably transmitted from one set of neurons to the next. The initial response of neurons to stimulus onset appears to have a similar form in different brain areas with a remarkably low jitter (Maršálek et al., 1997).
The mechanisms that produce the low jitter in neuronal firing times during transmission from one `layer' of neurons to the next can be readily understood. Noise and broad postsynaptic potentials tend to smear out initially sharp spike packets. If, however, the synaptic coupling is strong enough, then postsynaptic neurons will start firing already during the rising phase of their membrane potential. If, in addition, these neurons show pronounced refractory behavior, then firing will cease before the postsynaptic potentials have reached their maximum so that a sharp pulse of spike activity is generated. Refractoriness thus counteracts the effects of noise and synaptic transmission and helps to maintain precise timing.
In the following we show how the theory of population dynamics developed in
Chapter 6 can be used to provide a quantitative description of
the transmission of spike packets. We consider M pools containing N
neurons each that are connected in a purely feed-forward manner, i.e., neurons
from pool n project only to pool n + 1 and there are no synapses
between neurons from the same pool. We assume all-to-all connectivity between
two successive pools with uniform synaptic weights
![]() =
= ![]() /N;
cf. Fig. 9.15. In the framework of the Spike Response Model the membrane
potential of a neuron
i
/N;
cf. Fig. 9.15. In the framework of the Spike Response Model the membrane
potential of a neuron
i ![]()
![]() (n + 1) from pool n + 1 that has fired
its last spike at
(n + 1) from pool n + 1 that has fired
its last spike at ![]() is given by
is given by
![[htbp]
\centerline{\includegraphics[width=10cm]{pops.eps}}](img1547.gif) |
In contrast to the previous section, we explicitely take noise into
account. To this end we adopt the `escape noise model' (Section 5.3)
and replace the sharp firing threshold by a firing probability that is a
function of the membrane potential. The probability to find an action
potential in the infinitesimal interval
[t, t + dt) provided that the last
spike occured at ![]() is given by
is given by
| prob{spike in[t, t + dt) | last spike at |
(9.59) |
f (u) = 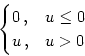 . . |
(9.60) |
With this probabilistic criterion for triggering spikes both spike train and
membrane potential become random variables. However, each pool is supposed to
contain a large number of neurons (N ![]() 1) so that we can replace the
population activity An in Eq. (9.61) by its expectation value which
is given by a normalization condition,
1) so that we can replace the
population activity An in Eq. (9.61) by its expectation value which
is given by a normalization condition,
| Si(t| |
(9.62) |
Simulation studies suggest that a pronounced refractory behavior is required
in order to obtain a stable propagation of a spike packet from one layer to
the next (Diesmann et al., 1999; Gewaltig, 2000). If neurons were allowed to fire more
than once within one spike packet the number of spikes per packet and
therewith the width of the packet would grow in each step. Therefore, we use a
strong and long-lasting after potential ![]() so that each neuron can fire
only once during each pulse. The survivor function thus equals unity for the
duration
so that each neuron can fire
only once during each pulse. The survivor function thus equals unity for the
duration
![]() of the afterpotential, i.e.,
sn(t,
of the afterpotential, i.e.,
sn(t,![]() ) = 1 for
0 < t -
) = 1 for
0 < t - ![]() <
< ![]() and
and
![]() being large as compared to the typical pulse width. Let us
denote by Tn the moment when a pulse packet arrives at pool n. We
assume that for t < Tn, all neurons in layer n have been inactive,
i.e., An(t) = 0 for t < Tn. Differentiation of Eq. (9.64) with
respect to t leads to
being large as compared to the typical pulse width. Let us
denote by Tn the moment when a pulse packet arrives at pool n. We
assume that for t < Tn, all neurons in layer n have been inactive,
i.e., An(t) = 0 for t < Tn. Differentiation of Eq. (9.64) with
respect to t leads to
Particularly interesting is the iteration that describes the amplitude of the spike packet. We will see below that the amplitude an in layer n as a function of the amplitude an-1 in the previous layer is independent of the shape of the spike packet, viz.,
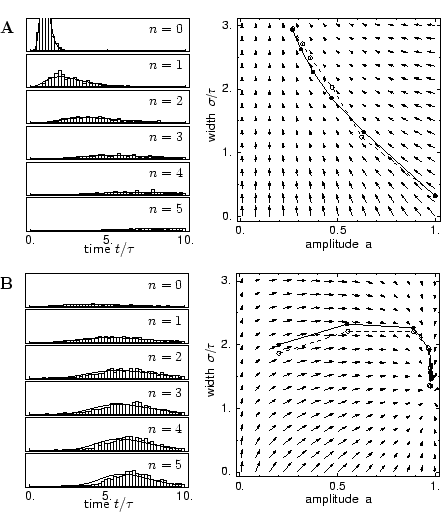 |
In the following we calculate the form of the spike packet in layer n as a
function of the form of the packet in layer n - 1. To this end we describe
the spike packet in terms of the first few moments, as outlined above.
In step (i) we assume that the activity
An-1(t) in layer n - 1 is
given by a gamma distribution with parameters
![]() and
and
![]() , i.e.,
, i.e.,
| An-1(t) = an-1 |
(9.66) |
The membrane potential un(t) in the next layer results from a
convolution of An-1 with the response kernel ![]() .
This is the only point where we have to refer
explicitely to the shape of the
.
This is the only point where we have to refer
explicitely to the shape of the ![]() kernel. For the sake of
simplicity we use a normalized
kernel. For the sake of
simplicity we use a normalized ![]() function,
function,
| (9.67) |
We want to approximate the time course of the membrane potential by a
gamma distribution
![]() . The
parameters9.2
. The
parameters9.2
![]() and
and
![]() are chosen so that the
first few moments of the distribution are identical to those of the
membrane potential, i.e.,
are chosen so that the
first few moments of the distribution are identical to those of the
membrane potential, i.e.,
In step (ii) we calculate the firing-time distribution that results from a membrane potential with time course given by a gamma distribution as in Eq. (9.71). We use the same strategy as in step (i), that is, we calculate the first few moments of the firing-time distribution and approximate it by the corresponding gamma distribution,
| An(t) |
(9.71) |
A combination of Eqs. (9.73) and (9.75) yields
explicit expressions for the parameters
(an,![]() ,
,![]() ) of the firing-time distribution in layer n as a
function of the parameters in the previous layer. The mapping
(an-1,
) of the firing-time distribution in layer n as a
function of the parameters in the previous layer. The mapping
(an-1,![]() ,
,![]() )
)![]() (an,
(an,![]() ,
,![]() ) is closely related to the neural
transmission function for pulse-packet input as discussed by
(Diesmann et al., 1999).
) is closely related to the neural
transmission function for pulse-packet input as discussed by
(Diesmann et al., 1999).
© Cambridge University Press
This book is in copyright. No reproduction of any part
of it may take place without the written permission
of Cambridge University Press.